I promised that rather than just bitching about crappy phage therapy papers I would offer some thoughts about applications where PT could be successful and have an impact. None of my suggestions will have the drama of PT swooping in to rescue a dying patient for whom doctors had given up all hope. But they will address unmet clinical needs that cause real suffering. And maybe, once we have figured out how to use phage effectively in a few chronic indications, we can start working on therapies for acute indications and start generating the dramatic plot lines so craved by science journalists.
Here’s suggestion #1: bacterial vaginosis.
It’s a disease that is known principally for causing bad smells and discomfort – rather than death and disability – and thus tends to be overlooked in the roster of unmet medical needs. But this is a mistake.
BV doesn’t kill anyone, at least not directly. But it increases the risk of pre-term births, spontaneous abortions, pelvic inflammatory disease, endometritis, and the acquisition and transmission of several sexually transmitted agents. And we are not talking about a few people here. BV is widespread, affecting about 27M American women each year. It is a serious disease and a significant burden on public health.
It meets the criteria I laid out for being amenable to phage therapy: current treatments, particularly those based on antibiotic therapy, are unsatisfactory; the problems of phage delivery and clearance are not so daunting; a single pathogen can be targeted.
It won’t surprise you to learn that the vagina has its own microbiome, comprised largely of Lactobacilli. These bugs normally secrete acids and hydrogen peroxide that keep the bad bugs under control. The bad bug that needs to be targeted for BV is Gardnerella vaginalis, an obligate anaerobe.
Historically there was some dispute as to whether Gardnerella is the principal pathogen for BV – it is found in healthy vaginas, and other bacteria are also positively associated with BV. These observations support the conclusion that the disease is polymicrobial. But a consensus seems to have emerged that Gardnerella is indeed the culprit: although not the sole pathogen, it initiates infection, competes directly with beneficial Lactobacilli, and sports an array of virulence factors that account for its pathogenicity. More indirectly, sexual transmission of BV also argues for a single causative agent, as is the case with other STDs.
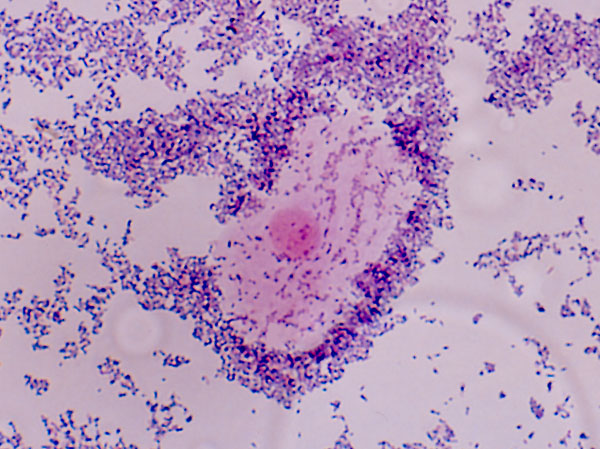 G. vaginalis cells coating a shed vaginal epithelial cell
G. vaginalis cells coating a shed vaginal epithelial cellThat said, BV should still be considered a microbiome disease, as it is the balance of Lactobacilli to Gardnerella, rather than the presence of Gardnerella that distinguishes disease from health. As such, we might expect to find that antibiotic use is a predisposing factor, but I can’t find any studies which confirm (or refute) that antibiotic use leads to BV.
What is more clear is that antibiotic therapy is less than satisfactory. Clindamycin and metronidazole are the first-line choices, with response rates of 70% and more. But relapse rates are high, and metronidazole has some unpleasant side effects such as nausea, and it induces violent barfing if you ingest even a tiny amount of alcohol.
Established infections form biofilms which reduce the efficacy of antibiotic therapy. Anti-biofilm agents have shown some value as adjuncts to antibiotics. Many phage, of course, sport biofilm-dissolving enzymes, a feature that makes them potentially well-suited for treating infections whose biofilms block the activity of antibiotics. A cocktail of anti-biofilm phage mixed with highly lytic phage would likely prove more potent than either alone, and simpler to administer than a cocktail of antibiotics mixed with anti-biofilm agents.
One obstacle in the development of PT for BV is that there are no Gardnerella phages. At least, none that I can find in the literature. But this is less of a problem than you might think. Finding phages is the easiest part of developing a phage therapeutic. I’m pretty sure that the only reason we don’t have Gardnerella phages is that no one has been looking.
Indeed, a genomic survey of clinical Gardnerella isolates finds over 400 different prophages integrated into their chromosomes. These are lysogenic phages that are mostly content to free-ride their bacterial host, and are not suitable as therapeutic agents. But it is implausible that all Gardnerella phages are lysogenic. Instead, it is likely that many lytic phages exist. And if they don’t exist, they can be selected from the existing prophages. One way or another, suitable phages will be found.
Delivery of therapeutic phage doses should be relatively straightforward. In systemic phage therapy applications, phage must be diluted into a large volume (the average adult has about 5 liters of plasma) from which it is rapidly cleared (the half-life of phage in plasma is just a few minutes). This means that maintaining a therapeutic concentration of phage is extremely challenging. No one has come close to solving this challenge; few studies even address it explicitly.
The vagina is a far more accessible compartment for drug delivery and it is likely that phage would persist for hours. Finding an optimal formulation for vaginal phage therapy would no doubt be a lot of work, but it would just be work, not something that requires a breakthrough. Like finding suitable phage, finding a suitable formulation is entirely doable.
There is some risk that a narrow-spectrum agent targeting only Gardnerella would be ineffective, and it is surely the case that it would not cure all infections. Even if Gardnerella is key to initiating infections, it does not necessarily follow that eliminating it also eliminates infections. This is probably the key technical risk for a BV phage therapy project. But it is very likely that an effective anti-Gardnerella agent would have significant therapeutic benefit in a large fraction of cases.
Thinking outside the antibiotic box a bit more, it would make sense to combine anti-Gardnerella PT with Lactobacillus-based probiotic therapy. That is, a therapy which narrowly targets a bad bug and replaces it with a good bug is likely to be much more effective than either approach alone.
A number of studies of probiotic treatment of BV, either alone or as an adjunct to antibiotic therapy, have been conducted. Although results are generally promising, the wide variation in protocols and formulations, and the small size of most studies leaves room for doubt. Still, results like this suggest that probiotics have clinical value and need to be investigated more thoroughly:
Despite the prevalence of antibiotic resistance, it is still true that antibiotic therapy works pretty well for most indications. Even if first-line therapy fails, second- or third-line therapies nearly always work. Antibiotics are perhaps the most successful medicines ever devised; it is a fantasy to expect that doctors are going to use alternatives like phage therapy as anything but a last resort.
The exceptions are those indications where experience shows that antibiotic therapy does not work so well. That is when doctors will be open to alternatives. Bacterial vaginosis meets this criterion. And so, unlike most infectious disease indications, phage therapy could plausibly compete with antibiotic therapy on the basis of greater efficacy and fewer side-effects. Rather than being a third- or fourth-line salvage therapy, it could be first-line. That seems to me a much better way to get PT into the clinic, rather than relying on a few one-off case studies.


1 thought on “Potential phage therapy application: bacterial vaginosis”