After decades of stagnation, I believe we are witnessing a renaissance of antibiotic discovery research.
There are two principal factors driving a resurgence in antibiotic discovery today: availability of public funding, and application of rational design and systems biology approaches.
For several decades now, antibiotics have been (and remain) a medium-risk/low-reward proposition for drug developers. Although anti-infectives (a class which includes antivirals) are more likely than other drugs to succeed in clinical testing, they are far less likely to make serious money once cleared for marketing (this is true of antibiotics, but not true of antivirals).
Here are the success rates for anti-infectives vs other drug categories:
From Clinical Development Success Rates 2006-2015
But sales are low, so it takes a long time to recoup R&D expenses:
From The grim prospect
And this is for the drugs that get approved. The 80% that don’t are just dead losses.
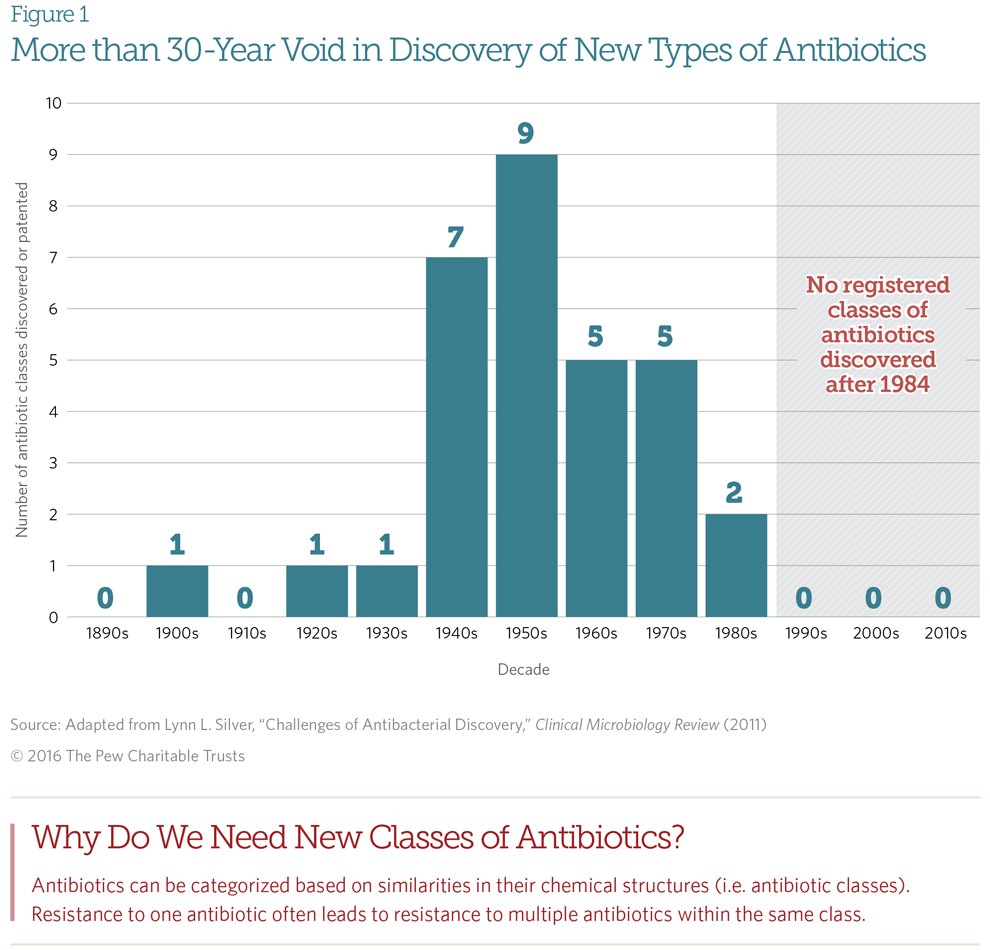
Big Pharma companies, with impeccable free-market logic, decided that antibiotic sales simply do not justify this risk and expense and cut these programs. Not surprisingly, the discovery of new antibiotics slowed, and the discovery of new classes of antibiotics stopped:
From A Scientific Roadmap for Antibiotic Discovery
Governments and NGOs, to their enormous credit, recognized this reality and stepped up direct funding efforts for antibiotic R&D. CARB-X is a multi-national consortium of government agencies and NGOs that is bringing real money to bear on antibiotic research – $500M has now been committed. That is enough to make a difference. The current list of grantees is here.
In addition to this new funding model, antibiotic discovery methods are finally entering the 21st century. The classic model from the Golden Age was a phenotypic screen: bacteria are exposed to some source of potential compounds, and those that stop bacterial growth are selected for further investigation and development. That’s how we got penicillin and streptomycin, and it’s even how we got teixobactin, a new compound that was the hot news in antibiotic discovery in 2015.
But there are limits and drawbacks to this approach. Big pharmas recently screened over 8 million different compounds without finding a single lead worth pursuing. And the phenotypic screening approach led to a very limited set of compounds that target only a few biochemical pathways: DNA synthesis, RNA synthesis, protein synthesis and cell-wall synthesis. Human cells (and especially mitochondria) share the first three pathways, sometimes leading to drug toxicities (e.g., ototoxicity for streptomycin). Limiting drug targets to a few pathways increases the likelihood of cross-resistance to new drugs.

From Antibiotic Classification & Mechanism
The exciting news is that omics technology, along with computationally-based drug design, and advanced cell engineering methods, are being applied to antibiotic discovery. These disciplines are being used to identify:
- targets that are critical to bacterial growth, thus ensuring drug effectiveness
- targets that are not readily changed or bypassed, and compounds that are not readily degraded or exported, thus reducing the risk of resistance
- targets unique to bacteria, thus reducing the risk of human toxicity
- drugs that are likely to penetrate the outer membrane of Gram-negative bacteria, a barrier which makes them much harder to treat.
For a couple of really nice examples of modern multidisciplinary approaches to antibiotic R&D, I recommend this paper that identifies likely targets in Klebsiella, and this one that engineered whole bacterial cells to use in screening compounds that specifically attack membrane formation pathways in Gram-negative bacteria.
I’ve written that the only thing really lacking to bring new antibiotics to the clinic is money. We are now starting to see the money – no thanks to the market – and will soon start seeing the drugs. We are entering a new golden age of antibiotics, one that is powered by the socialization of drug discovery.

1 thought on “New antibiotic R&D – the return of socialized drug development”