Antivirulence therapies were among the very first scientific medicines. In the 1880’s Emil von Behring and Kitasato Shibasaburo developed antiserum therapies for diphtheria and tetanus. These antisera, when injected into patients, neutralized bacterial toxins without killing the bacteria. They cut the case fatality rates for these terrible diseases in half, and von Behring (but not the darker-skinned Kitasato) was recognized with the first Nobel Prize in Medicine and Physiology in 1901.
Antiserum therapy had a number of problems as a medicine, and was quickly eclipsed by antibiotics when they became available. But with the rise of antibiotic resistance and a new appreciation of the microbiome, the case for antivirulence therapy as a substitute or complement to antibiotic therapy is compelling.
For example, Clostridium difficile-associated diarrhea kills nearly 30,000 Americans each year and is nearly always precipitated by antibiotic use. The standard of care is even more antibiotic treatment, but recurrence rates of 25% are common. A monoclonal antibody that neutralizes C difficile toxin cuts the recurrence rate to 60% of that seen with antibiotic therapy alone, and was recently cleared for clinical use by the FDA. Studies in mice show that this targeted therapy approach spares the gut microbiome, allowing it to recover more quickly. A number of other antivirulence therapies are in development (I wrote about some of them in New Scientist).
The scientific community is split on the issue of resistance to antivirulence therapy. The high-level argument against resistance development is that if you don’t kill the bugs, you don’t select resistant variants. The high-level counter-argument is that virulence factors provide a survival benefit, and neutralizing them will inevitably create selective pressure.
We are talking about biology here, so the answer is going to be complex and full of exceptions. However, theoretical and experimental considerations suggest a general pattern that might emerge:
- Virulence factors that promote the fitness of the bacterial community (public goods) are less likely to develop resistance to antivirulence therapy
- Virulence factors that promote the fitness of individual cells (private goods) are more likely to develop resistance to antivirulence therapy.
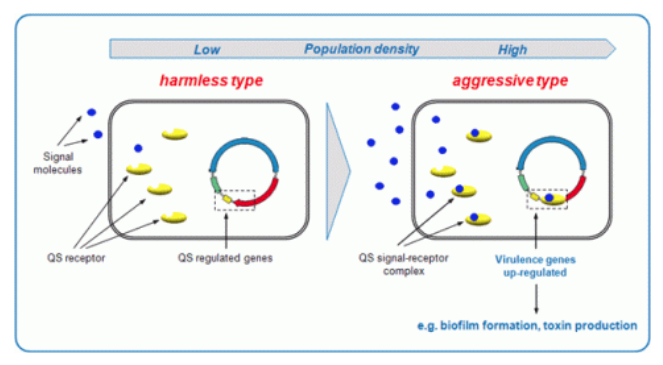
Examples of community-targeted antivirulence therapies include quorum-sensing inhibitors. Bacteria send out signalling molecules that tell them how many close relatives are nearby. If there are enough to constitute a quorum, these bugs will activate a set of genes that switch them from a state of peaceful commensalism to active virulence.
From Quorum Sensing and Biofilm | Advanced Healing
By contrast, adhesion inhibitors decrease the fitness of individual cells, and are known to lead to the selection of resistant variants.
Ultimately, the success of any antivirulence therapy will depend on a number of factors: the bacteria and virulence systems targeted, the type of infection, the duration and intensity of therapy, combination with other therapies such as antibiotics.
I suspect it will be a decade or more before antivirulence therapies really start to become effective and well-accepted. But the effort will be worthwhile – managing our bacteria rather than carpet-bombing them will leave us much healthier.