The inevitable has happened and a US patient was killed by a Klebsiella pneumoniae strain resistant to all available antibiotics. Just as inevitably, a swarm of news articles and blog posts appeared, claiming that the antibiotic apocalypse is now upon us and that we are beset by a plague of superbugs.
Not likely.
Medical and science journalists have failed miserably to draw a distinction between antibiotic resistance in bacteria and their ability to actually infect and sicken us, a property known as virulence. Here is Nicholas Bagley, writing for the New York Times, completely misrepresenting the origin and nature of antibiotic resistant bacteria:
Although most bacteria die when they encounter an antibiotic, a few hardy bugs survive. Through repeated exposure, those tough bacteria proliferate…
Bacteria do not acquire resistance by being stronger or hardier or tougher than antibiotic-susceptible bacteria. They become resistant by acquiring specific molecules that inactivate antibiotics, or they acquire molecular changes in the targets of antibiotic action. In no cases do these changes make the bacteria stronger, except in the specific sense of being able to resist antibiotics.
Think about it. Bacteria are constantly engaged in cutthroat competition for resources. The bug that grows fastest wins. If a mutation made bacteria stronger or hardier – and thus able to survive better and grow faster – wouldn’t it already have acquired this mutation?
Antibiotics disrupt core cell functions – DNA replication, RNA synthesis, protein synthesis, cell wall synthesis – that dictate the rate at which cells grow. These functions have been honed to a high degree of efficiency by billions of years of natural selection. Changes are not likely to result in better performance. Antibiotic resistance should make bugs weaker, not stronger.
That’s the theory. How does it hold up in experiment and clinical observation?
There is a substantial body of scientific literature which shows that antibiotic resistance imposes a fitness cost. A typical experiment is to take two strains of bacteria that are identical save for a resistance trait, mix them together where they have to compete for resources, and see which strain wins. The answer, with few exceptions, is that the antibiotic susceptible strain wins. On average, a resistant strain is about 88% as fit as a susceptible strain. This pattern holds over a wide variety of antibiotics and bacteria:

And the more resistances a bug acquires, the less fit it becomes:
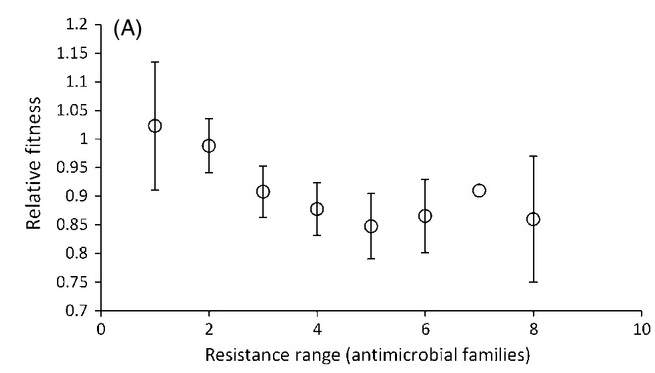
“Fitness” however, is a concept that is wholly dependent on context. What is fit in one environment may not be fit in another. Lab results are informative, but what we really care about are clinical outcomes. If antibiotic resistant bacteria like MRSAs and CREs and ESBLs are truly superbugs, then we would expect to see them sicken and kill more patients than antibiotic-susceptible strains.
Acquiring a resistant pathogen, especially a multi-resistant pathogen, increases the risk of death, consistent with the notion that these bugs are superbugs. But a closer look at the data reveals two trends that argue against increased virulence associated with resistance: (1) patients that acquire multi-drug resistant infections tend to be already much sicker than patients infected with susceptible strains[1] [2] ; (2) when patients with MDR infections receive appropriate antibiotic therapy (though many do not), they are less likely to die[3] [4] . The typical victim of an MDR “superbug” infection is old, and already in the ICU for some other condition such as kidney or lung failure or advanced cancer. These bugs take the old and weak, not the young and healthy.
If resistant strains are in fact less hardy than susceptible strains, then we would expect to see them outcompeted when their one selective advantage – antibiotic resistance – is removed. This is typically the case[5] . The story of MRSA control in Denmark is instructive. When these strains first appeared in the 1960s, the Danish health authorities instituted a strict program of antibiotic stewardship. Deprived of the competitive advantage provided by indiscriminate antibiotic use, the resistant strains could not keep up with the susceptible ones, and by 1975 MRSA infections became – and have remained – rare in Denmark.
In the UK and the US there were no such controls. MRSA strains persisted in hospitals, where they soon accounted for half or more of all S. aureus infections. But failure to take decisive action led to an even worse outcome. The relatively feeble hospital MRSA strains of the 1960s began acquiring compensatory mutations that made them more robust and hardy, and they broke out of hospitals in the 1990s. One of these “community” strains (USA300) also acquired a new virulence factor, Panton-Valentin Leukocidin, that kills neutrophils. Thus armed, MRSA became a true superbug, one that is not merely resistant to a range of antibiotics, but is also able to inflict severe infections on otherwise strong and healthy adults.
This is the real danger. The MDR bugs we have now can be suppressed – although never eliminated – with the boring and tedious work of infection control, antibiotic stewardship, rapid microbiology testing and public health measures. Yes it would be nice to have new antibiotics, but without an attitude change new antibiotics will become compromised within a few years. Calling every new strain of multidrug-resistant bacteria a superbug may generate clicks, but does nothing to advance the public’s understanding of the threat they pose. And without support for public health measures and antibiotic stewardship, weak bacterial strains will become strong and the superbug apocalypse will indeed be upon us.
Footnotes
[1] Risk factors associated with the isolation of colistin-resistant gram-negative bacteria: a matched case-control study.
[2] Risk factors of carbapenem-resistant Klebsiella pneumoniae infections: a matched case control study.
[3] Impact of carbapenem resistance on the outcome of patients’ hospital-acquired bacteraemia caused by Klebsiella pneumoniae.
[4] Risk factors for bacteriuria with carbapenem-resistant Klebsiella pneumoniae and its impact on mortality: a case-control study.
[5] The fitness costs of antibiotic resistance mutations
